Abstract
Patients with relapsed or refractory chronic lymphocytic leukemia (R/R CLL) receiving venetoclax (VEN) are at risk of tumor lysis syndrome (TLS). This may be mitigated by implementing a 5-week ramp-up dosing schedule, use of antiuricemic agents and continuous hydration in high-risk patients. Currently, there is a lack of real-world data on the incidence of TLS in routine clinical care of R/R CLL patients treated with VEN in Canada. This is particularly the case amongst heavily pretreated patients progressing on prior ibrutinib who would be considered at high risk for aggressive disease and subsequent TLS.
The DEVOTE study is a Canadian multicenter open-label study examining the real-world characteristics of patients with R/R CLL, their management with special attention to TLS mitigation measures, and their health care resource utilization and quality of life.
Eligible patients were adults who had provided written informed consent, had CLL and had received ≥1 prior line of therapy, and whose physician had decided to prescribe VEN in routine clinical practice. Those participating in an interventional study were excluded. Assessments included baseline and concomitant medication use, TLS risk, clinical and laboratory assessments at baseline and ramp up visits, and measures taken to mitigate TLS.
This interim analysis included 69 patients, 64 of whom completed ramp up phase. Median (range) age was 71 (39-91) years; 74% were male; 18/57 cases had del17p and 19/24 were documented as IgVH unmutated. Baseline TLS risk was low for 21 patients, medium for 37, and high for 11. Median time from last relapse to baseline assessment was 5.9 weeks. Patients had undergone a median (range) of 2 (1-7) prior lines of therapy with 63 previously treated with ibrutinib; 36 discontinued due to disease progression, 23 for intolerance, and 4 for other reasons. Ibrutinib was the last prior line of therapy in 59 patients, of whom 18 continued to take it concomitantly during VEN dose ramp up (for a median of 19 days).
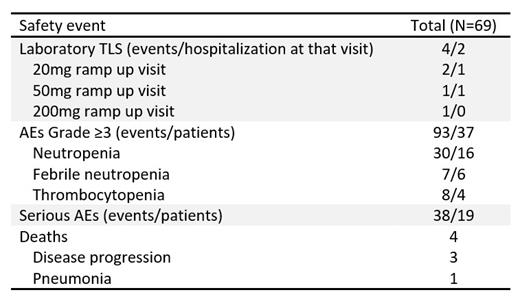
Four laboratory TLS events (per Howard's criteria, conservatively modified to include total calcium in place of ionized or corrected calcium) were reported in 4 patients (Table). The patients were medium (3) or high (1) TLS risk. The 2 patients with laboratory TLS at the 20mg ramp up visit (both medium TLS risk) had CLL progression on ibrutinib and were taking it concomitantly at the time of laboratory TLS. No patient developed renal dysfunction, nor required rasburicase therapy or dialysis, and all patients continued on VEN therapy. No cases of clinical TLS were reported.
Antiuricemic agents were administered preemptively to 62 patients, starting a median of 7.5 days before the 20mg ramp up visit and 67 (including all high TLS risk patients) received these agents concomitant with VEN for a median of 29 days. Two patients (both medium TLS risk) did not receive an antiuricemic agent at any time during the study; neither had a measurement of elevated uric acid during the study. Uric acid > 475 μmol/L was reported at baseline for 7 patients, including 1 patient (not hospitalized) who experienced a TLS event at the 20mg ramp up visit and 2 patients with high TLS risk, neither of whom received rasburicase or experienced a TLS event.
Overall, 25 patients were hospitalized at least once, all of whom were hospitalized for the 20mg ramp up visit with numbers diminishing for subsequent visits. The 44 patients who completed ramp up as out-patients included 2 patients with high TLS risk. The product monograph recommends hospitalization for high TLS risk or medium TLS risk with CrCl <80mL/min. Overall, 23 patients met this criteria, of whom only 12 were hospitalized. Thirteen patients who did not meet the criteria were hospitalized for the 20mg visit at the physician's discretion. The only characteristic associated with hospitalization was TLS risk.
Most patients (87%) reached a VEN dose of 400 mg daily, 75% of whom did so in ≤5 weeks. Safety profile was consistent with previous reports (Table).
In this heavily pre-treated population including a majority of patients with disease progression on ibrutinib, few cases of laboratory TLS and no clinical TLS were reported during initiation of VEN. No patient required rasburicase and some patients deemed at high risk for TLS were safely treated as outpatients. As the study was performed soon after VEN approval in Canada, patient characteristics and TLS mitigation strategies may have evolved with time.
Banerji: Janssen: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy; Gilead: Consultancy; Roche: Consultancy; Lundbeck: Consultancy. Aw: AbbVie: Honoraria; AstraZeneca: Honoraria. Bull: AbbVie: Current Employment, Current equity holder in publicly-traded company. Fournier: AbbVie: Current Employment, Current equity holder in publicly-traded company. Johnson: Merck: Consultancy; Roche: Consultancy, Honoraria; BMS: Consultancy; Seattle Genetics: Consultancy; Gilead: Consultancy; AbbVie: Consultancy, Research Funding. Laferriere: AbbVie: Honoraria; Alexion: Honoraria; Amgen: Honoraria; Astellas: Honoraria; BMS: Honoraria; Gilead: Honoraria; Leo Pharma: Honoraria; Janssen: Honoraria; Novartis: Honoraria; Roche: Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Teva: Honoraria. Lembo: AbbVie: Current Employment, Current equity holder in publicly-traded company. Pelizon: AbbVie: Current Employment, Current equity holder in publicly-traded company. Peters: AbbVie, Incyte: Consultancy, Honoraria. Owen: Roche: Honoraria, Research Funding; Servier: Honoraria; AbbVie: Honoraria, Research Funding; Genentech: Research Funding; Incyte: Honoraria; Merck: Honoraria; Gilead: Honoraria; AstraZeneca: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Pharmacyclics: Research Funding.